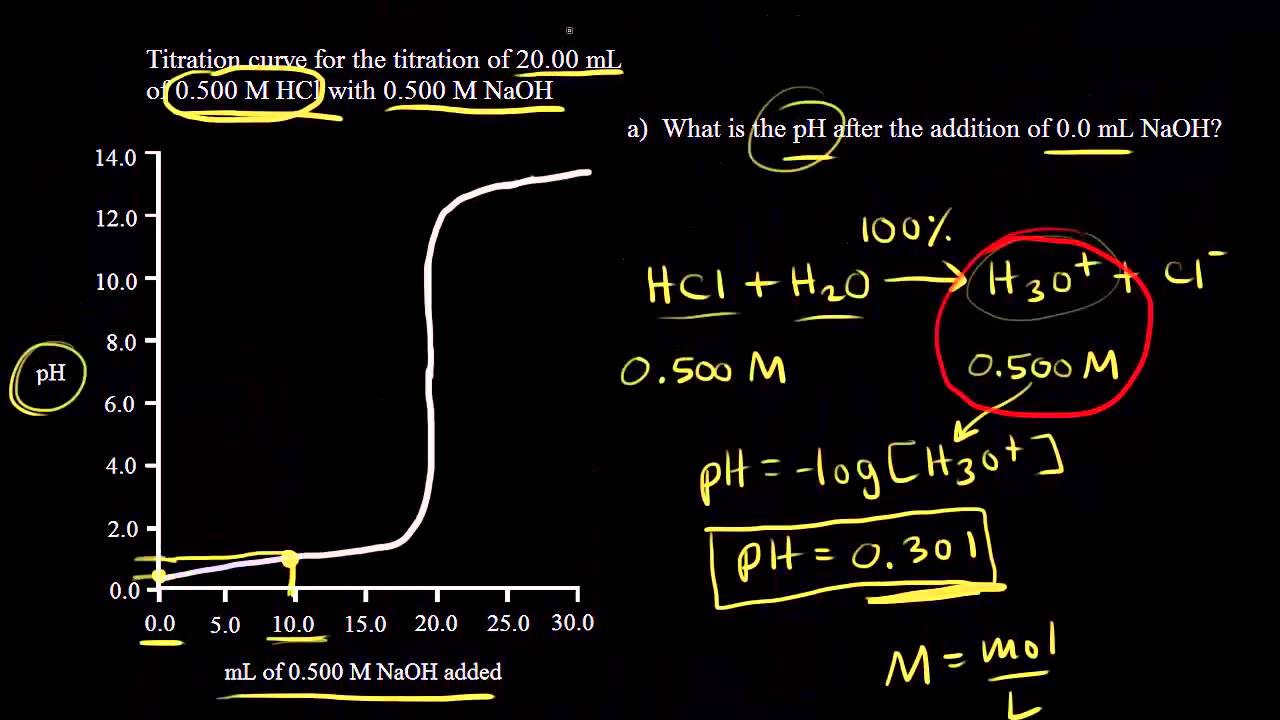
To neutralise 20 ml of M/10 sodium hydroxide, the volume of M/20 hydrochloric acid required is - YouTube
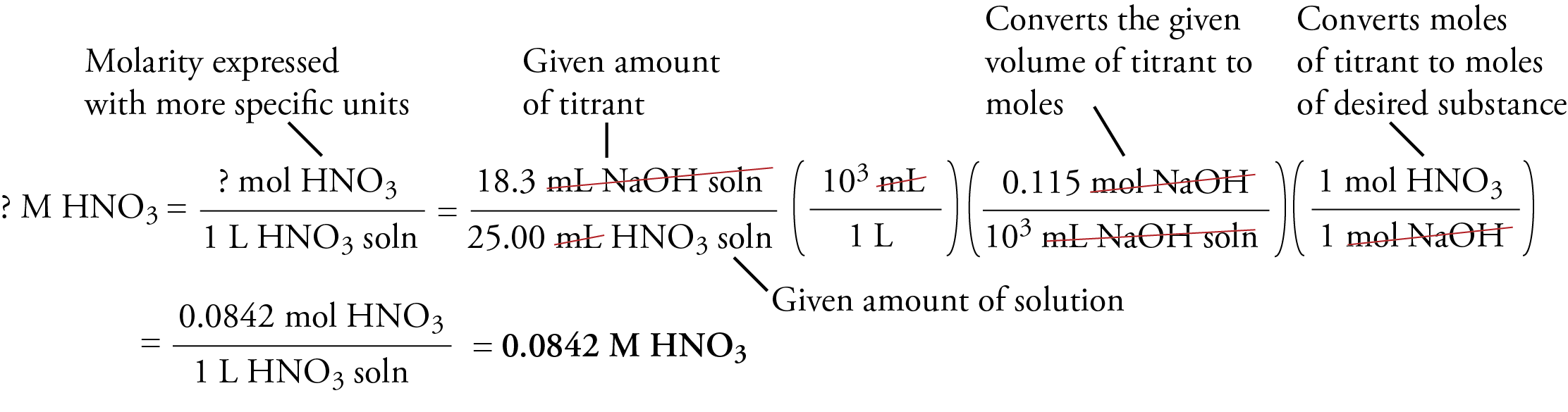
A 10.0 mL sample of 0.250M HCl (aq) is titrated to the end point with a 14.0 ml sample of NaOH(aq). What is the molarity of the NaOH (aq)? - Quora
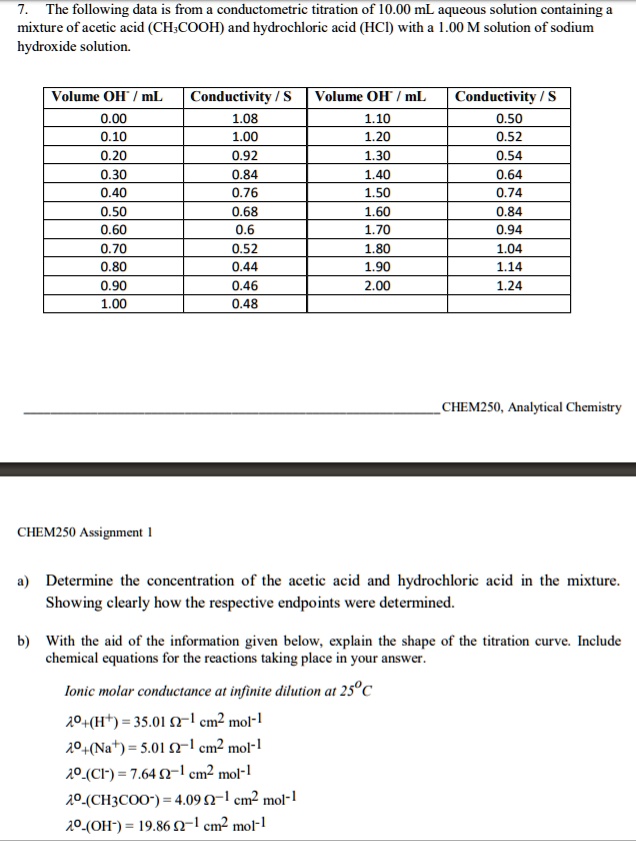
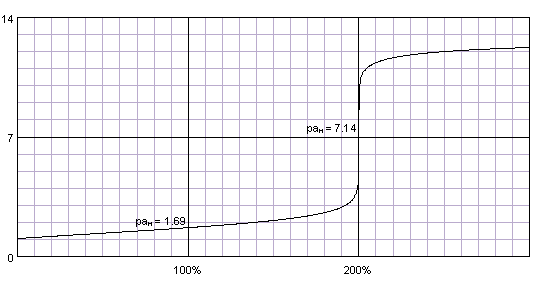
SOLVED: The following data is from conductometric titration of 10.00 mL aqueous solution containing mixture of acetic acid (CHCOOH) and hydrochloric acid (HCI) with 1.00 M solution of sodium hydroxide solution Volume
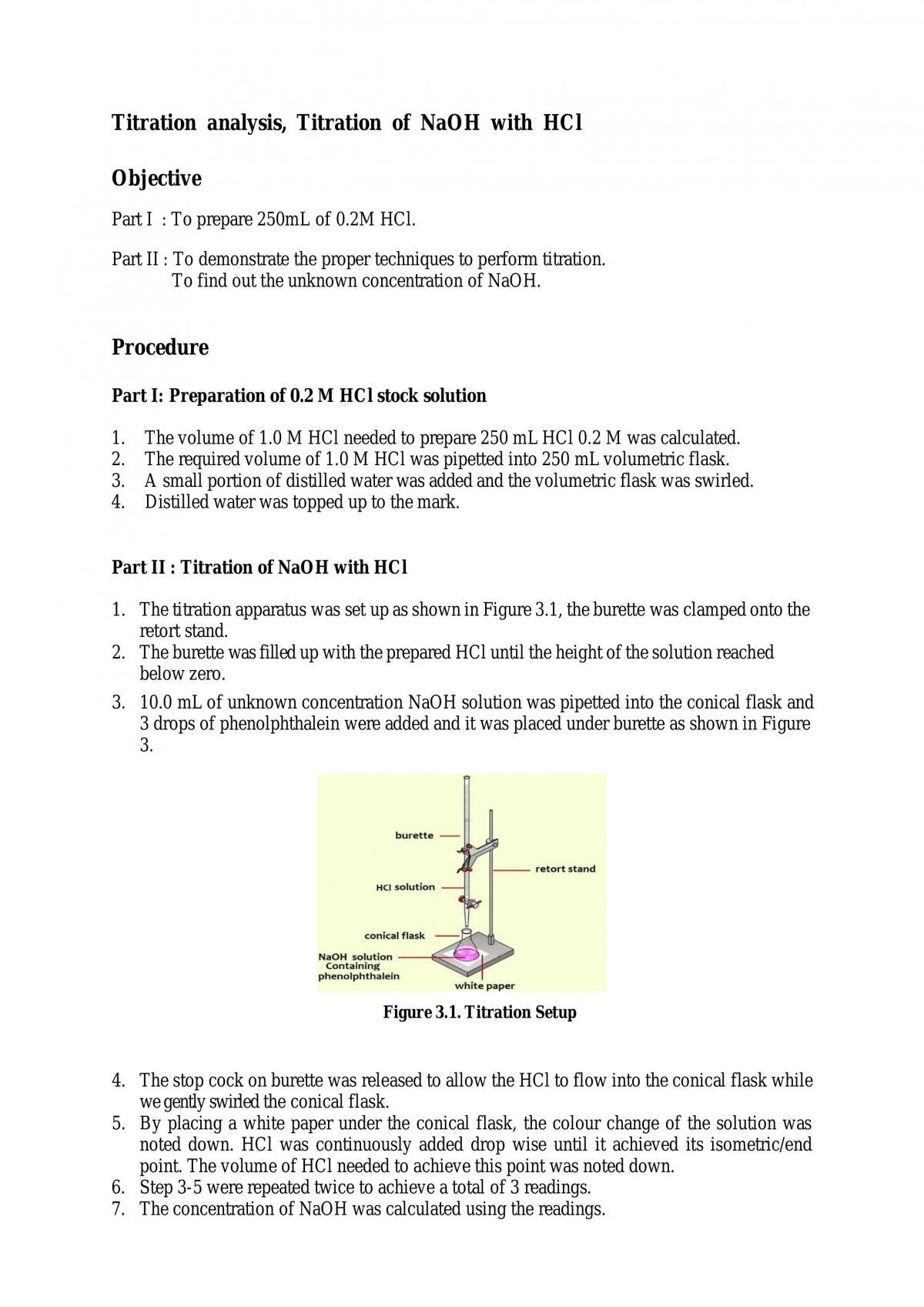
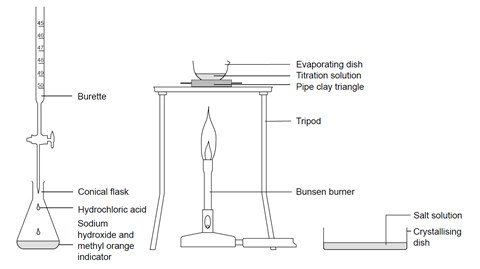
Titration of Sodium Hydroxide with Hydrochloric acid | FSC107 - General Chemistry Laboratory - XMUM | Thinkswap