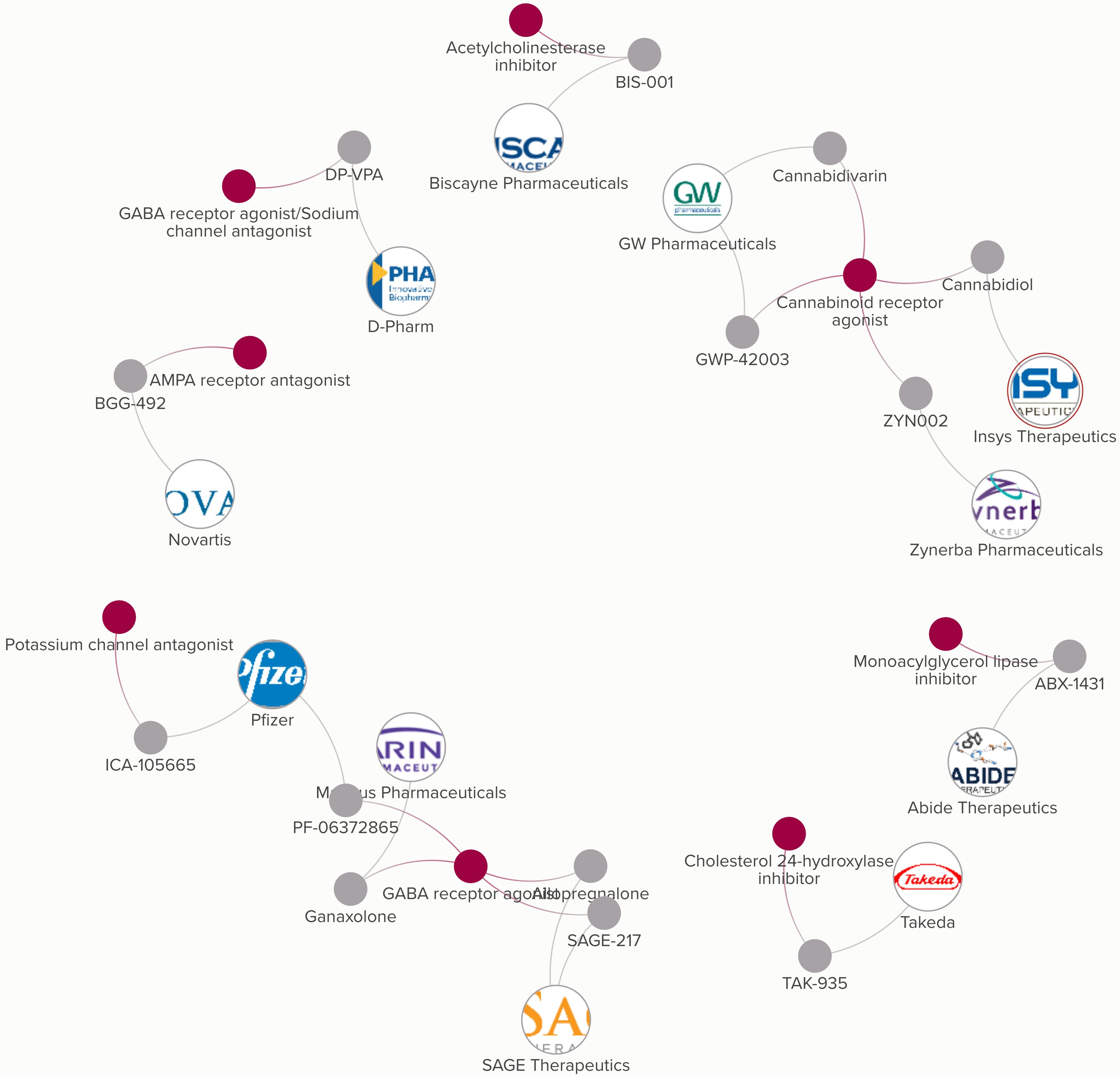
Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration | Business Wire
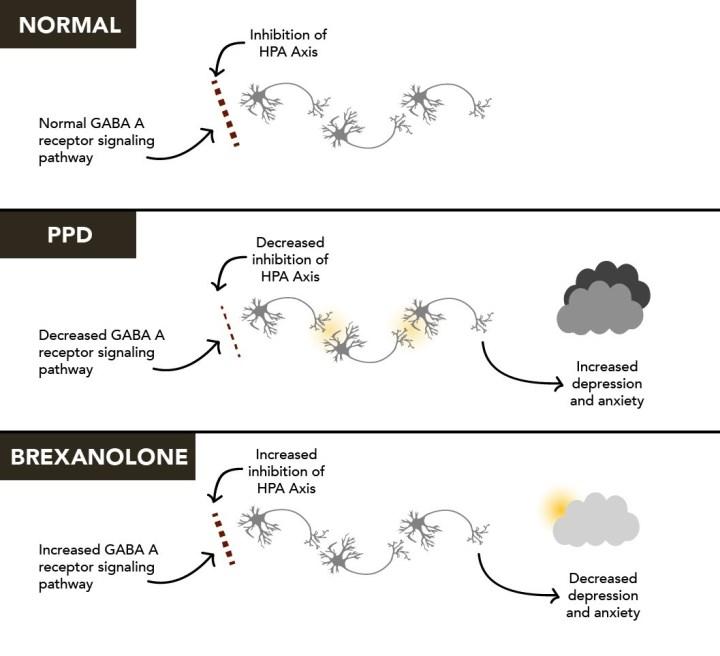
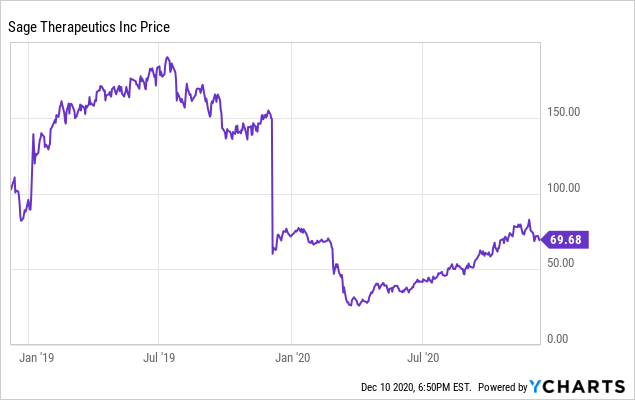
Sage Therapeutics: Zuranolone's Multi-Billion Dollar Market Opportunity More Than Justifies Company Valuation (NASDAQ:SAGE) | Seeking Alpha

Sage Therapeutics Reports Topline Results from Pivotal Phase 3 MOUNTAIN Study of SAGE-217 in Major Depressive Disorder | Business Wire

Biogen and Sage Therapeutics Complete Rolling Submission of New Drug Application for Zuranolone in the Treatment of Major Depressive Disorder and Postpartum Depression | Biogen

Sage Therapeutics Announces Development Plan for Zuranolone (SAGE-217) Following Breakthrough Therapy Guidance Meeting with the U.S. Food & Drug Administration-CliniExpert
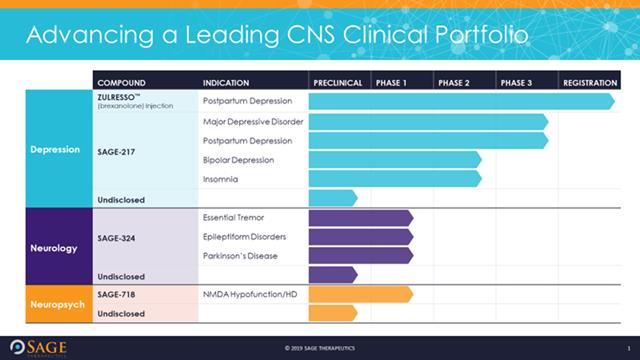
Sage Therapeutics Announces Second Quarter 2018 Financial Results and Highlights Pipeline and Business Progress
Sage Therapeutics Forges $575 Million Deal With Shionogi to Market Depression Drug in Parts of Asia | BioSpace

Sage Therapeutics and Biogen Initiate Rolling Submission of New Drug Application (NDA) to U.S. Food and Drug Administration for Zuranolone for the Potential Treatment of Major Depressive Disorder (MDD) | Business Wire
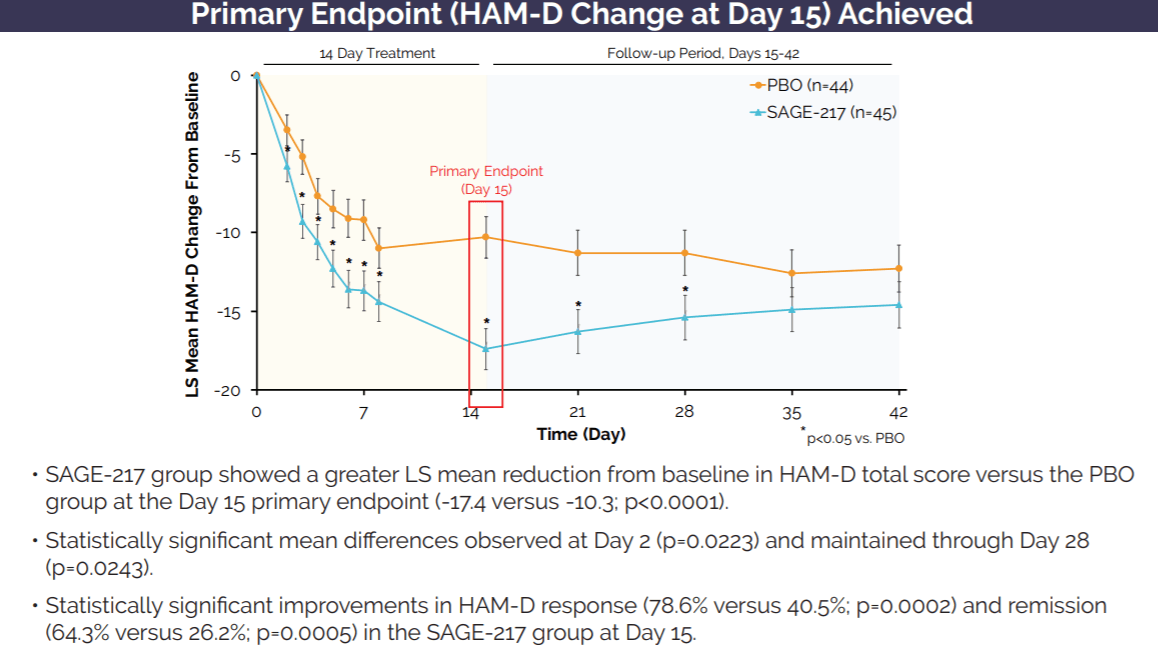
Sage Therapeutics: Zuranolone's Multi-Billion Dollar Market Opportunity More Than Justifies Company Valuation (NASDAQ:SAGE) | Seeking Alpha
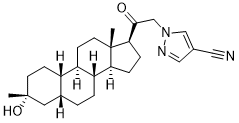
Sage Therapeutics Receives FDA Breakthrough Therapy Designation for SAGE-217 for the Treatment of Major Depressive Disorder - Chemdiv

Sage Therapeutics Reports Topline Results from Pivotal Phase 3 MOUNTAIN Study of SAGE-217 in Major Depressive Disorder

Breakthrough Therapy, PRIME and Sakigake: A Comparison Between Neuroscience and Oncology in Obtaining Preferred Regulatory Status - Elena Tomaselli Muensterman, Yijia Luo, Jonathon M. Parker, 2019